Thankfully, few medical devices have to be recalled because it has been discovered that they were defective, but when this does happen it is usually big news. Although it is normally the Federal Food & Drug Administration (FDA) that has the final authority to order a recall, more often the company that manufactures the device recalls them under pressure from lawsuits or because of widespread public recognition that there are significant problems with the application of the device.
Most recently, controversy about defective devices has included certain metal to metal hip replacement products and hernia and vaginal meshes.
When the FDA issues a notice to the manufacturer advising it that there is a perceived problem with the product, it will either be to correct the problem or recall the product until the device is classified as safe.
The FDA uses three classes of recall which are used to define the level of seriousness of the defective device. The least serious recalls fall into Class III. At this level, the device is not regarded as dangerous enough to cause injury, illness or death. Class II recalls may involve products that cause some kind of temporary health problem, while Class I recall products may cause serious injury or death.
Occasionally, the manufacturer may try and fight a recall issued by the FDA, but the agency has the authority to order a recall under a 21 CFR 810.
Once a recall has been ordered, or a product has been assigned a recall notice, the FDA will keep a record on its database and from time to time put out a notice or press release advising the public of the classification of the product.
Metal to metal hip replacement products under medical and legal scrutiny
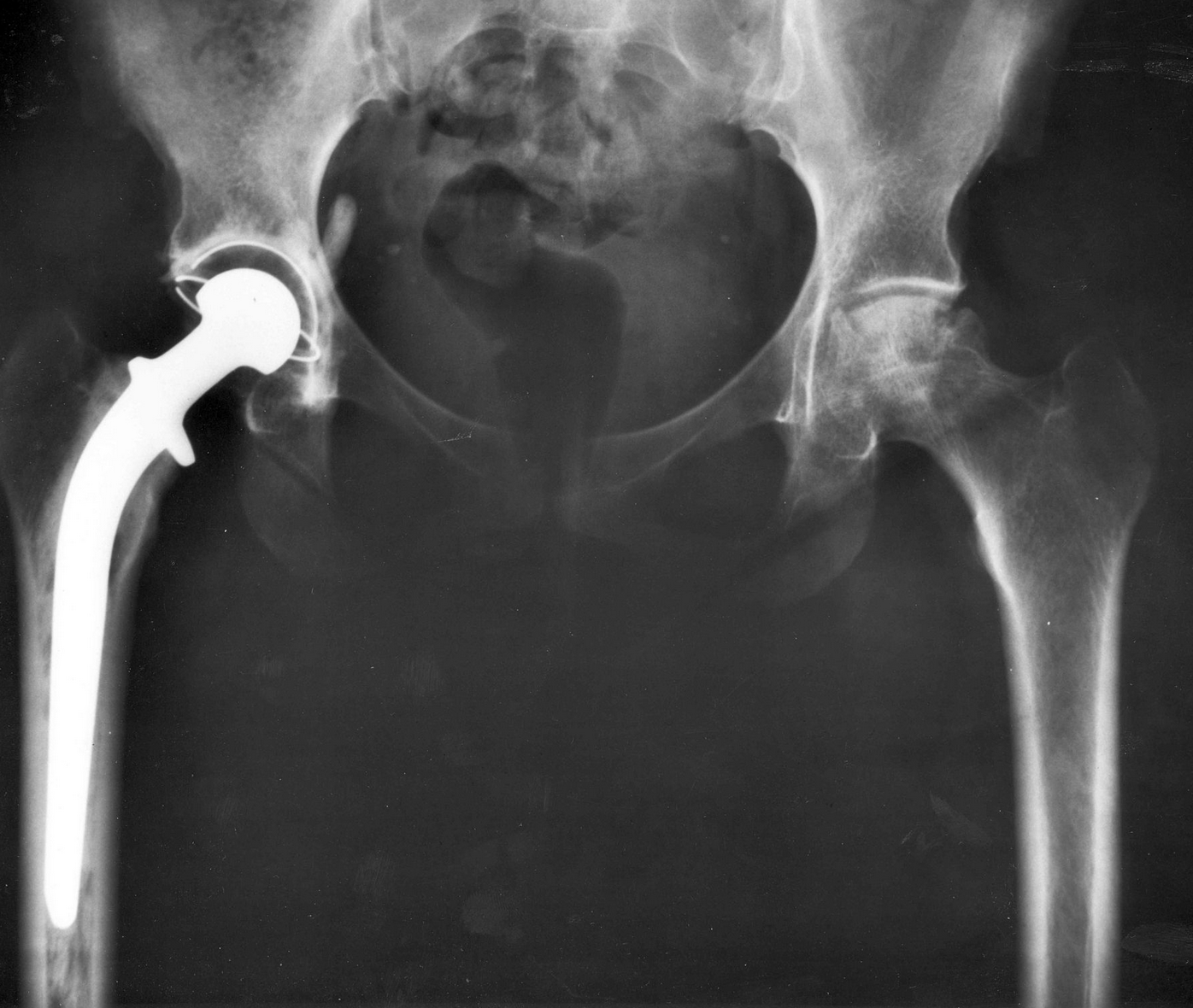
Some of the most contentious products in recent times have been a number of hip and knee replacement products that use metal devices. At first sight these devices seem to provide a strong and permanent solution to those requiring a major joint replacement, but it appears that there has been a problem with the material used.
At present, there are three different manufacturers of hip replacement products that are involved in lawsuits regarding the safety of their products. These companies are Stryker, DePuy Synthes and the British Smith & Nephew. Stryker and DePuy Synthes are both subsidiaries of the huge implant and medical drug manufacturer Johnson & Johnson, which has its own lawsuits against it because of talcum powder products it manufactures. The lawsuits are still ongoing despite the fact that the products under contention have already been recalled.
The three companies named above all produce metal hip replacement components made from cobalt and chromium.
Stryker manufactures a product called the LFIT V40 Femoral Head; DePuy Synthes manufactures the Pinnacle Hip System and Smith & Nephew’s product is the Birmingham Hip Resurfacing System.
The FDA acknowledges that the three hip replacement technologies share with other older hip replacement technology certain defects: bone loss, device fracture, bone fracture, device loosening, infection and joint dislocation. In addition, the metal components cause even more problems, the medical science of which is not yet perfectly understood.
The most serious problem appears to be from the break-up of the cobalt / chromium alloy into particles under friction at the joint. The particles ionize and cause local as well as more remote problems due to a process called metallosis, which is basically a form of heavy metal poisoning.
The lawsuits all allege that the three manufacturers have failed to carry out sufficient testing and research to eliminate potential danger to health before the products were released for use by hip replacement surgeons.
As of the time of writing of this blog, there were more than 9,000 product liability lawsuits before the courts against the three companies, with by far the majority of them against DePuy Synthes. The damages claimed run into the billions of dollars.
Why a defective product recall should get your attention
Unfortunately, some manufacturers release products that can hurt us or even kill us because they have not been tested properly. Even if you are using one of these products and have not yet noted any defects, the sooner it is noted and rectified the better. Keep an eye on any defective product recalls in the news, especially if you think they concern you or a member of your family. If you live in or around Nashville, you can contact a defective product liability attorney at the Keith Williams Law Group to discuss your legal options.

Leave A Comment